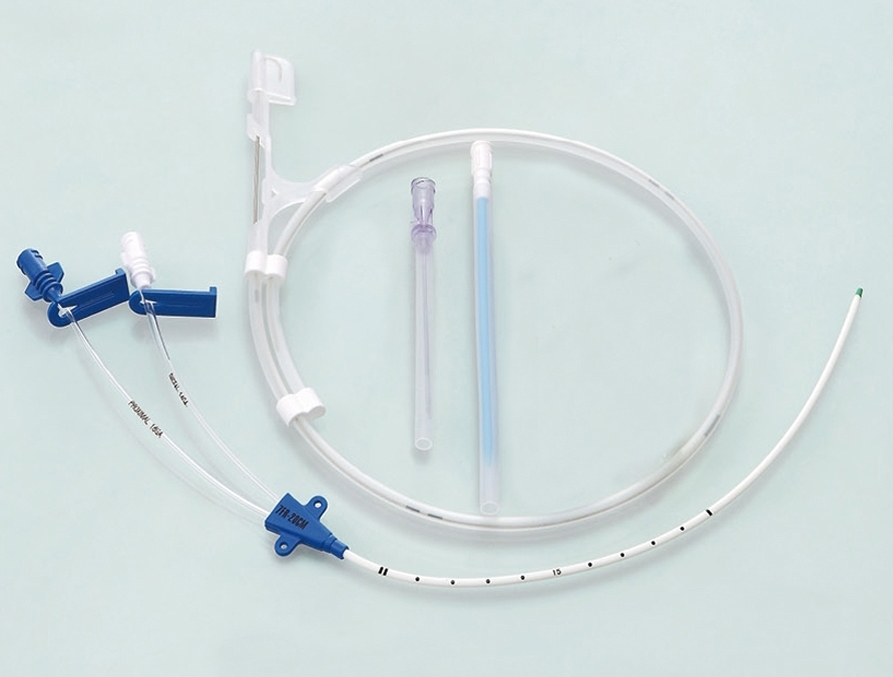
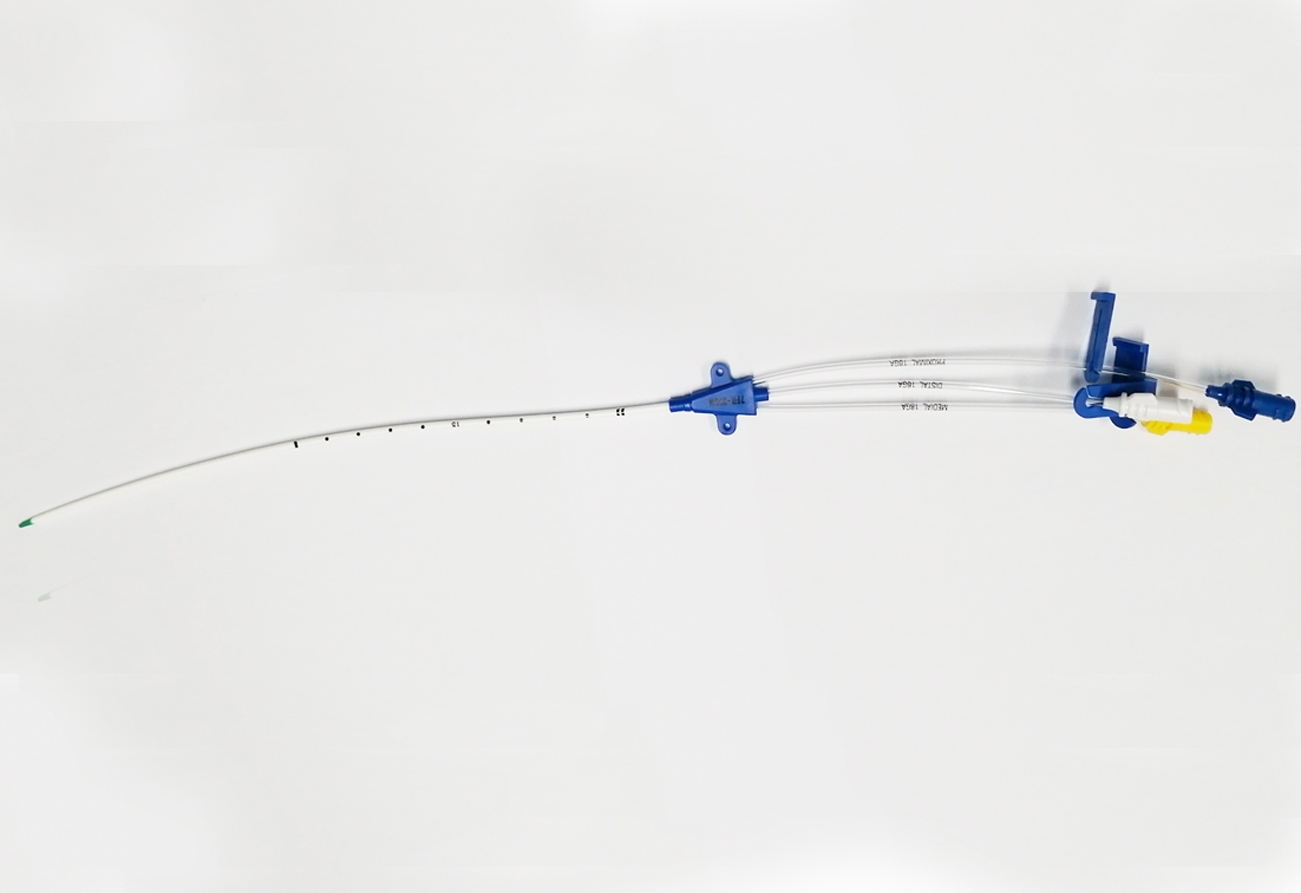
The ABLE® Central Venous Catheters (CVC) are sterile, single-use only polyurethane catheters designed to facilitate infusion therapy in a critical care environment. They are available in variety of lumen configurations, lengths, French and Gauge sizes. The multi-lumen variants provide dedicated lumens for infusion therapy, pressure monitoring and venous sampling. The CVC are packaged along with the components and accessories for insertion with Seldinger technique. All products are sterilized by ethylene oxide.
Intended Use and Indication
Warnings and Precautions
Contraindications and Complications
Order Information
Device Lifetime
SSCP
Advertising Review Filing Number
Intended Use:
The Central Venous Catheter Kit is used for providing vascular access.
The Catheter is surgically penetrated at three optional puncture points depending on the clinical requirement with the Seldinger Technique. The Insertion Sites could be :
- Internal jugular vein;
- Subclavian vein;
- Femoral vein.
Indication:
The CVC Kit may be applicable to one of the following therapies:
- Monitor of central venous pressure;
- Continuous or discontinuous venous infusion;
- Blood sampling or blood transfusion.
Note:The catheter
is intended for children, adolescents, and
adults.
Warnings:
1. The catheter is to be inserted and removed only by a suitably qualified physician or nurse;
2. The clinical benefit of the use of CVCs must be evaluated against the recognized risks and complications of the procedure.
3. Do not use if the packaging has been previously opened or damaged, or ifany part of the catheter is missing or damaged.
4. Observe aseptic technique at all times when handling the catheter and/or the insertion components and in accordance with standard hospital protocols.
5. Some disinfectants used at the catheter insertion site contain solvents that can weaken the catheter material. Alcohol, acetone, and polyethylene glycol can weaken the structure of polyurethane materials. These agents may also weaken the adhesive bond between the catheter stabilization device and skin. It is recommended to use Chlorhexidine with Alcohol (CHG-Alcohol) as the preferred disinfectant for skin disinfection and catheter maintenance during central venous catheterization.
WARNING: Do not use acetone on the catheter surface. Do not use alcohol to soak the catheter surface or allow alcohol to dwell in a catheter lumen to restore catheter patency or as an infection prevention measure. Do not use polyethylene glycol-containing ointments at the insertion site. Take care when infusing drugs with a high concentration of alcohol. Allow the insertion site to dry completely before skin puncture and before applying the dressing.
6. Do not apply undue force on the guidewire to minimize the risk of possible breakage.
7. Do not apply excessive force in removing the guidewire or catheter. If withdrawal cannot be easily accomplished, a visual image should be obtained and further consultation requested.
8. Do not resterilize.
9. Do not re-insert a partially or completely withdrawn needle.
10. Do not use for high-pressure infusion.
11. It is prohibited to use haemostatic forceps to clamp the extension tubes and hubs.
12. In case of thrombus in the lumen, rinse by force is not allowed; try to suck the clog by syringe first, if it doesn’t work, use blood clot dissolver.
WARNING: Only anexperienced physician is allowed.
13. Any serious incidents related to the device should be reported to
the competent authorities of the member states where the manufacturer, user,
and/or patient are located.
14. Do not reuse. The physical integrity and performance of the device may be compromised if reused. Reusing poses risks, including but not limited to: Causing infection; if serious, it may result in death; the needle tube and dilator may become blunt, leading difficulties in catheterization; decreased catheter flowrate; device blockage or leakage; damage to the surface of the device causes vascular injury, etc.
Precautions:
1. Confirm the position of the catheter tip by X-ray.
2. Use only aluer lock (threaded) connector and syringes with the catheter, and do not over-tighten.
3. Insert the guidewire into the blood vessel from the flexible end.
4. Please avoid damaging the catheter or personnel with sharp tools (including scalpel, needles, etc).
5. The catheter can tolerate 300kPa positive pressure and 80kPa negative pressure. It is not indicated for power injection.
6. The catheter does not contain any additives or coatings.
7. The intended usage time of the injection cap shall not exceed 24 hours.
8. The central venous catheter is MRI safe, whichdoes not contain any metal components.
Contraindications:
1. Hypersensitivity to device materials.
2. Infection or a cut wound around the puncture area.
3. Dysfunction of blood coagulation.
4. During the anticoagulant treatment.
5. Symptoms of inadaptability to puncture operation, such as Pneumothorax, venous sclerosis.
6. Abnormal or unclear anatomical situation at the penetration area, such as severe emphysema, or obvious inadaptability from a previous operation.
Complications:
The clinical benefit of the use of CVCs must
be evaluated against the recognized risks and complications of the procedure, which include, but are not limited to:
1. Infection, necrosis of the puncture point
2. Extravasation injury
3. Bleeding/hematoma
4. Air embolism
5. Cardiac arrhythmia
6. Thrombus
7. Pneumothorax
8. Arterial puncture
9. Hemothorax
10. Occlusion
|
Number of Lumen |
O.D. |
Effective Length/cm |
|
|
Ga / Fr |
mm |
||
|
Single |
14Ga |
2.00 |
10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
16Ga |
1.70 |
10, 13, 15, 16, 20, 25, 30, 40, 45, 60, 80 |
|
|
18Ga |
1.40 |
5, 6, 8, 10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
20Ga |
1.15 |
5, 6, 8, 10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
22Ga |
0.90 |
5, 6, 8, 10, 13, 15, 16, 20, 30, 40, 45, 60 |
|
|
24Ga |
0.65 |
5, 6, 8, 9, 10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
Double |
4Fr |
1.40 |
5, 6, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
5Fr |
1.70 |
5, 6, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
7Fr |
2.40 |
5, 8, 10, 13, 14, 15, 16, 20, 25, 30, 35, 40, 45, 60 |
|
|
8Fr |
2.70 |
10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
8.5Fr |
2.85 |
10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
Triple |
4Fr |
1.35 |
4, 5, 6, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
4.5Fr |
1.50 |
5, 6, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
5Fr |
1.70 |
5, 6, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
5.5Fr |
1.85 |
5, 6, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
6Fr |
2.00 |
5, 6, 8, 9, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
7Fr |
2.40 |
5, 8, 10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
7.5Fr |
2.50 |
10, 12, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
8.5Fr |
2.85 |
10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
|
|
Quad |
8.5Fr |
2.85 |
10, 13, 15, 16, 20, 25, 30, 40, 45, 60 |
1. OD of 20Ga, 22Ga, 24Ga, 4Fr and 4.5Fr are intended for children (< 9 years old) use.
2. OD of 18Ga, 5Fr and 5.5Fr are intended for adolescents (9 ~ 18 years old) use.
3. The flow rate of the catheter can be found in the Instruction for Use of the product or via consulting the sales representative.
4. The major components of the product package include central venous catheter, guidewire, dilator and puncture needle, etc.
Lifetime:
It is possible to be inserted inside the body for no more than 30 days. If the duration exceeds 30 days, it may cause the risk of combining the catheter and the inside tissue, which results in a serious incident. It is not recommended to use the product for more than 30 days. The Central Venous Catheter Kit is not directly involved in the treatment of the disease.Guangdong Advertising Approval No. [2025] 007108