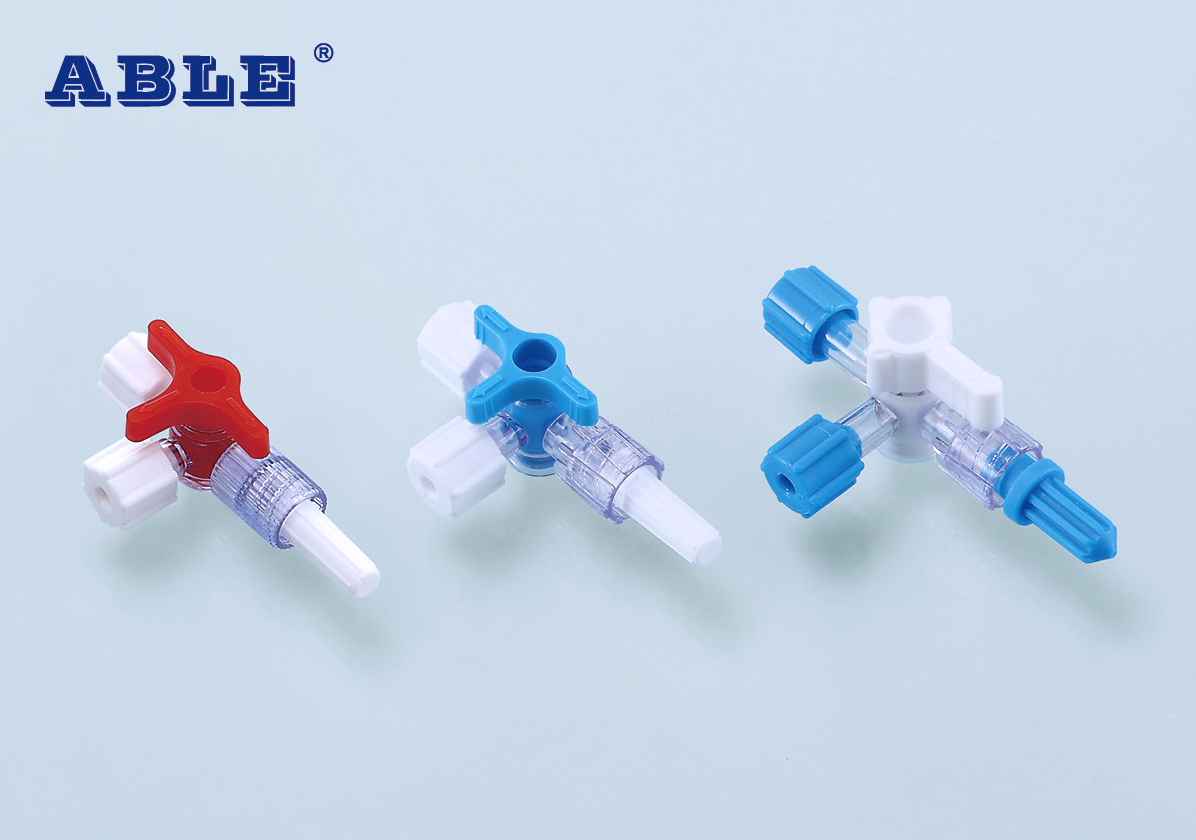
The ABLE® Disposable Stopcock is a sterile and single use device, which is made of medical grade polycarbonate, polyurethane and polyethylene materials. It consists of the base, spinning handle, female Luer, protective caps, extension tube and manifolds. The device is sterilized with ethylene oxide.
Intended Use
Order Information
Warning
Contraindications
The stopcock with extension tube is available in Normal and Pressure type; the stopcock manifold is available in form of 2 or 3 manifolds.
1. The use of the device must comply with the relevant operating standards and regulations of the medical department, and is limited to trained medical personnel only;
2. Please check the single package before use. Must not use if the package is opened or damaged;
3. All channels should be kept clean and sterile;
4. The shelf life of this device is 3 years.
5. The stopcock with pressure extension tube can be connected to the pressure infusion set to extend the pressure monitoring pipeline. Other models and specifications are prohibited from being used in high-pressure injection systems.
6. This device is only intended for one-time use, and repeated use increases the risk of cross infection. Strict aseptic operation is required during use. Dispose of waste products according to hospital or environmental protection department regulation after use.
7. When there is blockage or leakage during the correct use of the device, medical personnel should promptly stop treatment and replace the device for the patient.
8. Before starting the infusion of the device, it is necessary to confirm the tightness of the connection.
9. The use of infusion sets without threaded interfaces can easily lead to lose connections, leakage, and other situations. It is recommended to use infusion sets with threaded interfaces.
10. After tight connection, do not turn the stopcock counterclockwise before use to prevent accidental loosening or detachment, which may cause liquid leakage.
11. If the device is tightened too tightly, there may be a tendency for cracks to appear under the contact of infusion solution or medication.
12. When using the product in oncology, anesthesia, and other places, close attention should be paid to whether the medicine poses a risk of damage to the device.
13. Sediments of incompatible substances in the blood or coagulation and physicochemical properties of the connecting site may lead to the risk of cracking and difficult disassembly of the device.
14. Due to the sudden change in temperature required for treatment, there is a risk of device cracking and difficulty in disassembly.
15. To avoid the risk of device cracking, leakage, and loosening during use, multiple devices should be avoided from being used in series. Suggest using manifolds one.
16. The device is prone to damage under the erosion of medicine such as cyclosporine, nimodipine, etoposide, propofol, cyclophosphamide, lipids, and sodium phenytoin.
17. The stopcock should be properly fixed during use, especially for children, the elderly, irritable and emotionally unstable patients. Patrol should be strengthened, and if necessary, auxiliary methods should be used to fix it to avoid loosening and leakage during use.
18. To ensure stable operation and avoid problems such as looseness or difficulty in twisting, please hold the device with both hands for operation.