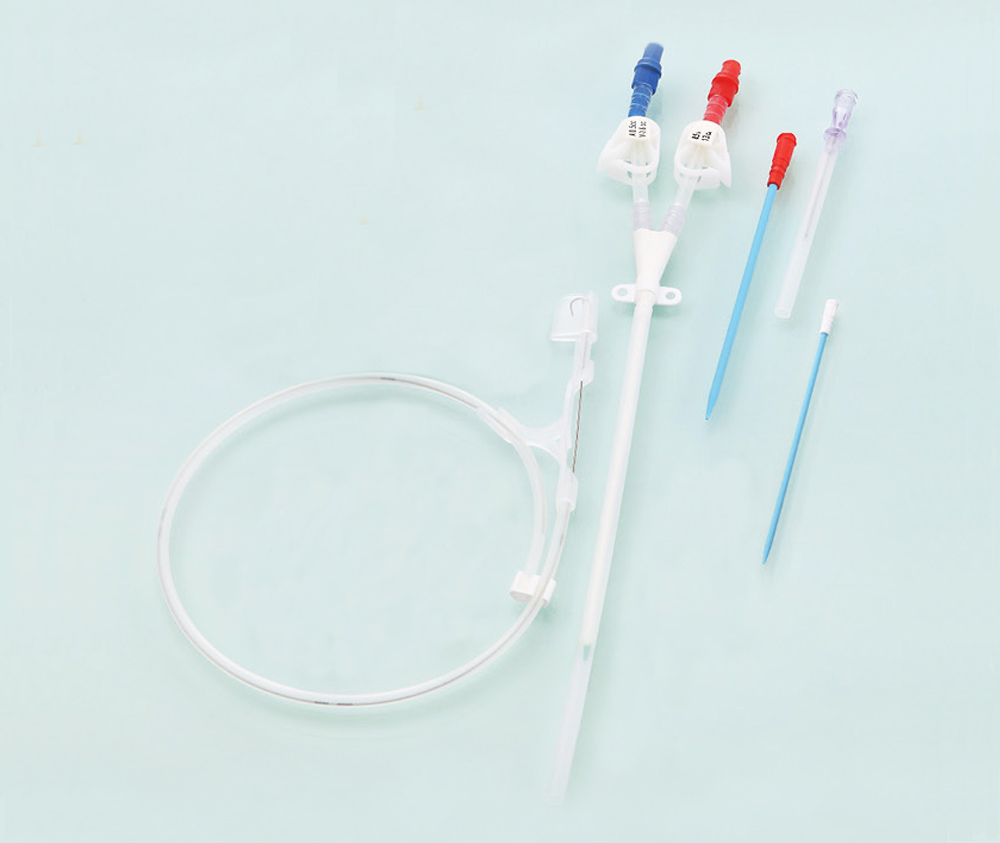
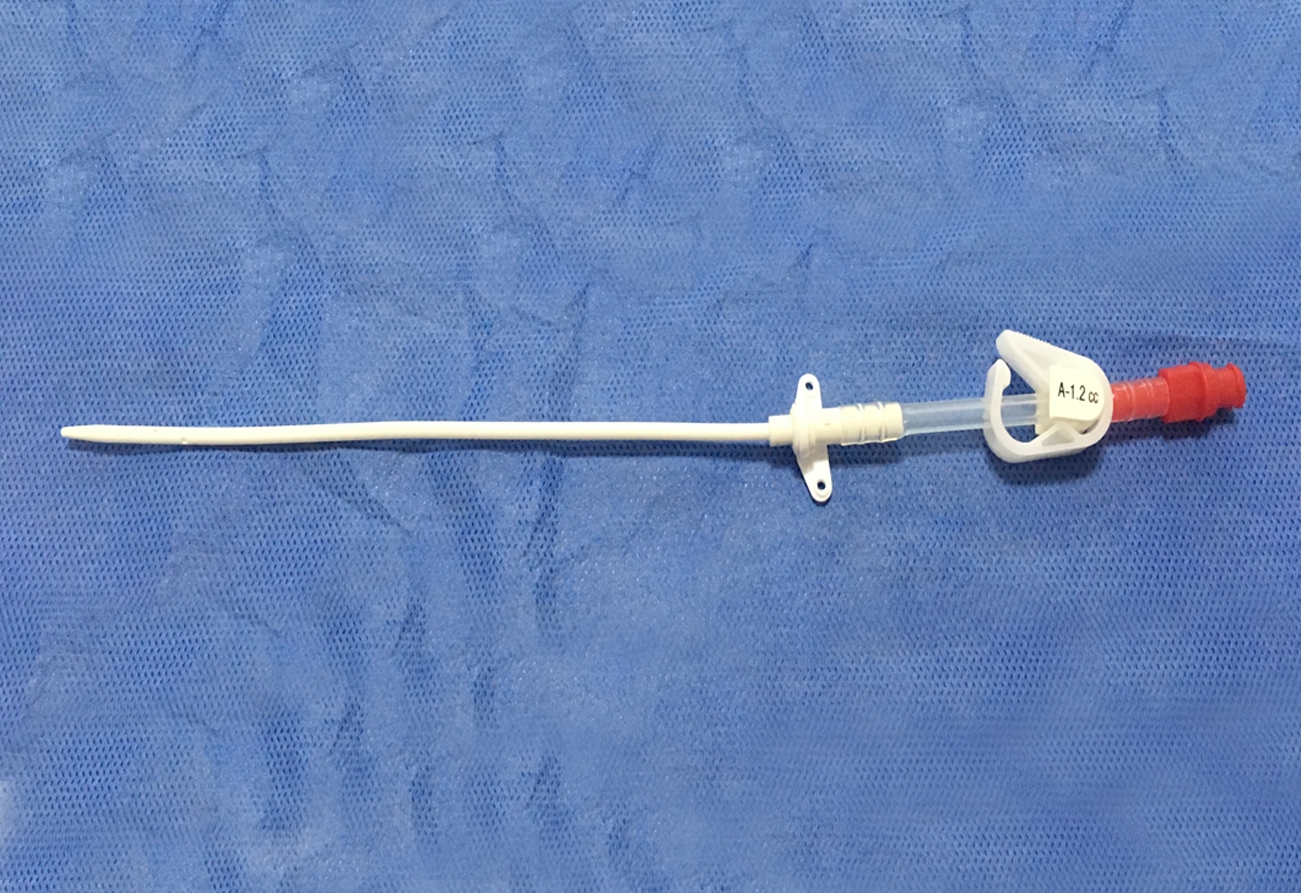
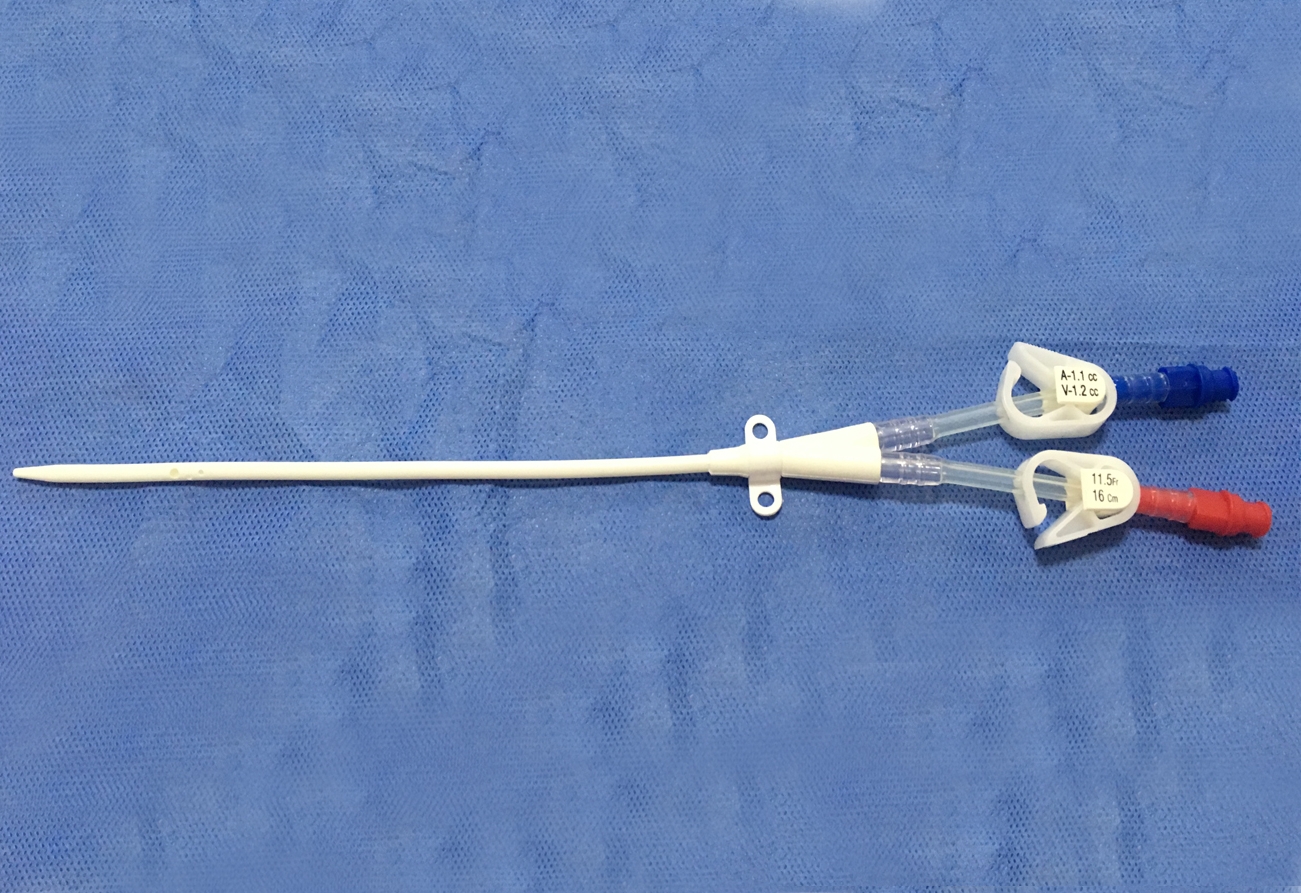
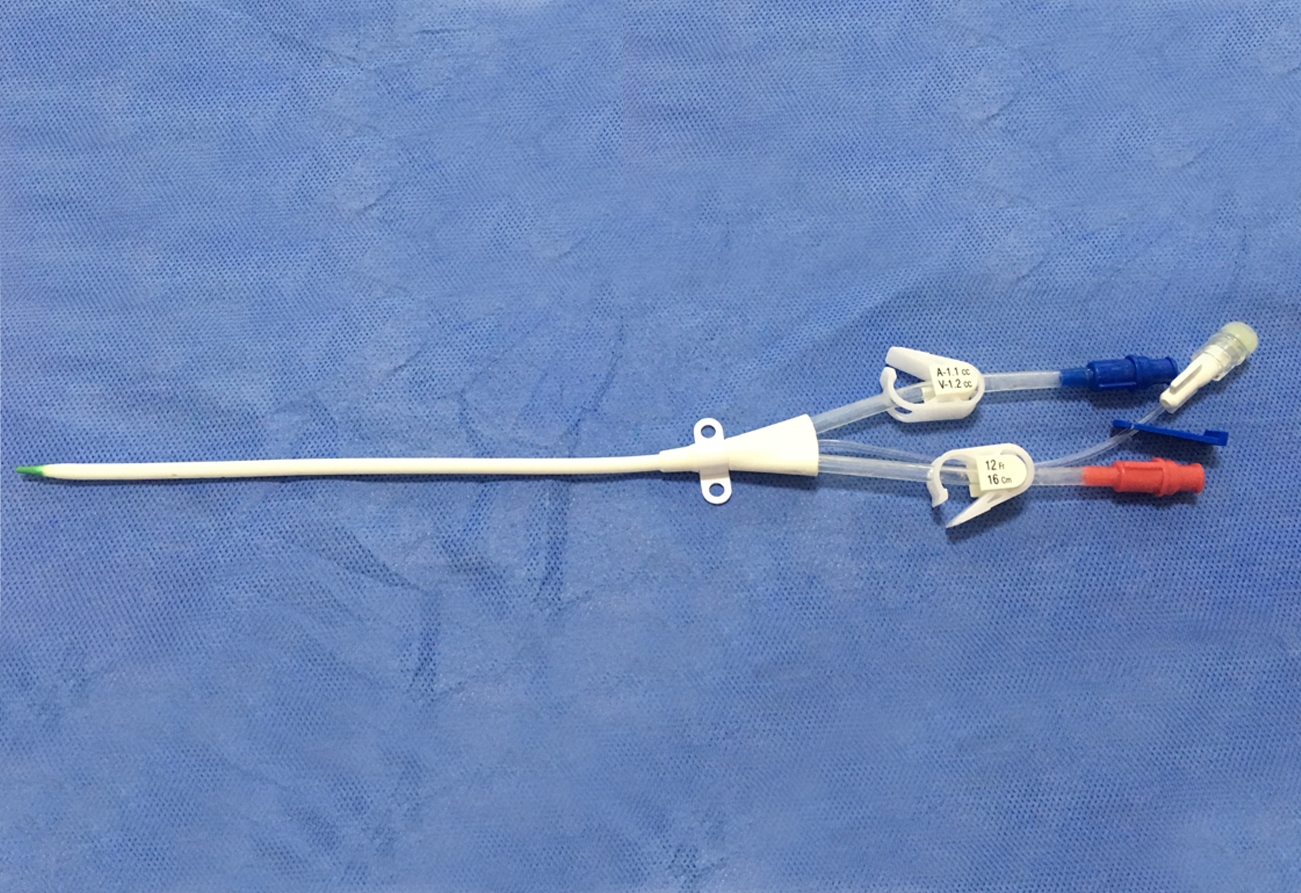
The ABLE® Haemodialysis Catheters (HDC) are sterile, single-use only polyurethane catheters designed to build a temporary blood connection between the patient and the dialysis machine for acute and short-term dialysis. They are available in variety of lumen configurations, lengths, French sizes. The multi lumen variants provide dedicated lumens for haemodialysis therapy, infusion therapy, pressure monitoring. The HDC are packaged along with the components and accessories for insertion with Seldinger technique. All products are sterilized by ethylene oxide.
Intended Use
Contraindications
Complications
Order Information
Advertising Review Filing Number
The ABLE® Haemodialysis Catheters may be
applicable to the one of following therapy:
- To supply the temporary blood vessel access in haemodialysis treatment;
- Monitor of central venous pressure;
- Continuous or discontinuous
venous transfusion.
The Catheter is surgically penetrated into three optional puncture points depended on the clinical requirement with Seldinger Technique. The Insertion Sites are :
1. Infection or cut wound around the puncture area.
2. Dysfunction of blood coagulation.
3. During the anticoagulant treatment.
4. Symptoms of inadaptability to puncture operation, such as Pneumothorax, vein sclerosis.
5. Abnormal or unclear anatomical situation at the penetration area, such as sever emphysema, obviously inadaptability from previous operation
1. The catheter body is manufactured from medical-grade polyurethane (TPU) with excellent biocompatibility.
2. Multiple lumen configurations to meet diverse clinical requirements.
For contraindications or precautions, refer to the instruction manual.
|
Number of Lumen |
O.D. |
Effective Length / mm |
|
|
Fr |
mm |
||
|
Single |
7Fr |
2.40 |
100, 130, 135, 150, 160, 200 |
|
8Fr |
2.70 |
100, 130, 135, 150, 160, 200 |
|
|
Double |
6.5Fr |
2.20 |
50, 80, 100, 130, 160 |
|
8.5F |
2.85 |
50, 80, 100, 110, 120, 130, 150, 160, 170, 200 |
|
|
10Fr |
3.40 |
80, 100, 120, 130, 150, 160, 170, 200 |
|
|
11.5F |
3.80 |
100, 120, 130, 135, 150, 160, 200, 240, 250 |
|
|
12Fr |
4.00 |
100, 120, 130, 135, 150, 160, 200, 240, 250 |
|
|
14Fr |
4.70 |
130, 150, 160, 190, 200, 240, 250 |
|
|
Triple |
12Fr |
4.00 |
130, 150, 160, 200, 240,250 |
The major components of the product package include central venous catheters, guidewire, dilator and puncture needle, etc.
For more information, please contact your sales representative or call +86-757-89959515.
Guangdong Provincial Medical Device Approval No. [2025]007127