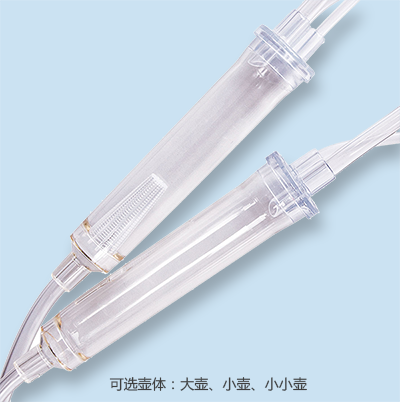
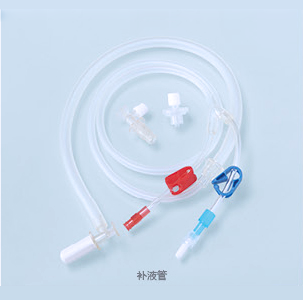
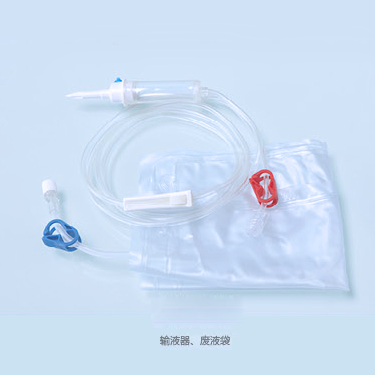
The ABLE® Disposable Blood Line is connected with other medical devices to establish extracorporeal circulation channel outside human body in the process of hemodialysis. The material of this product is medical-grade PVC. Tubes of this product are soft and transparent, it is covenient and credible to connect with fistula needle and dialyzer.
Intended Use and Indication
Ordering information
Contraindications
Warning
The Disposable Blood Line is intended to establish blood circulating path outside human body. It is sterile, single use and intended to be used by trained and experienced professionals.
Indications:
The Disposable Blood Line is used to connect with other hemodialysis devices to establish blood circulating path outside human body to form a hemodialysis system for the acute and chronic hemodialysis therapy.
No absolute contraindications.
Warning:
1. Prior to use, read the instruction carefully.
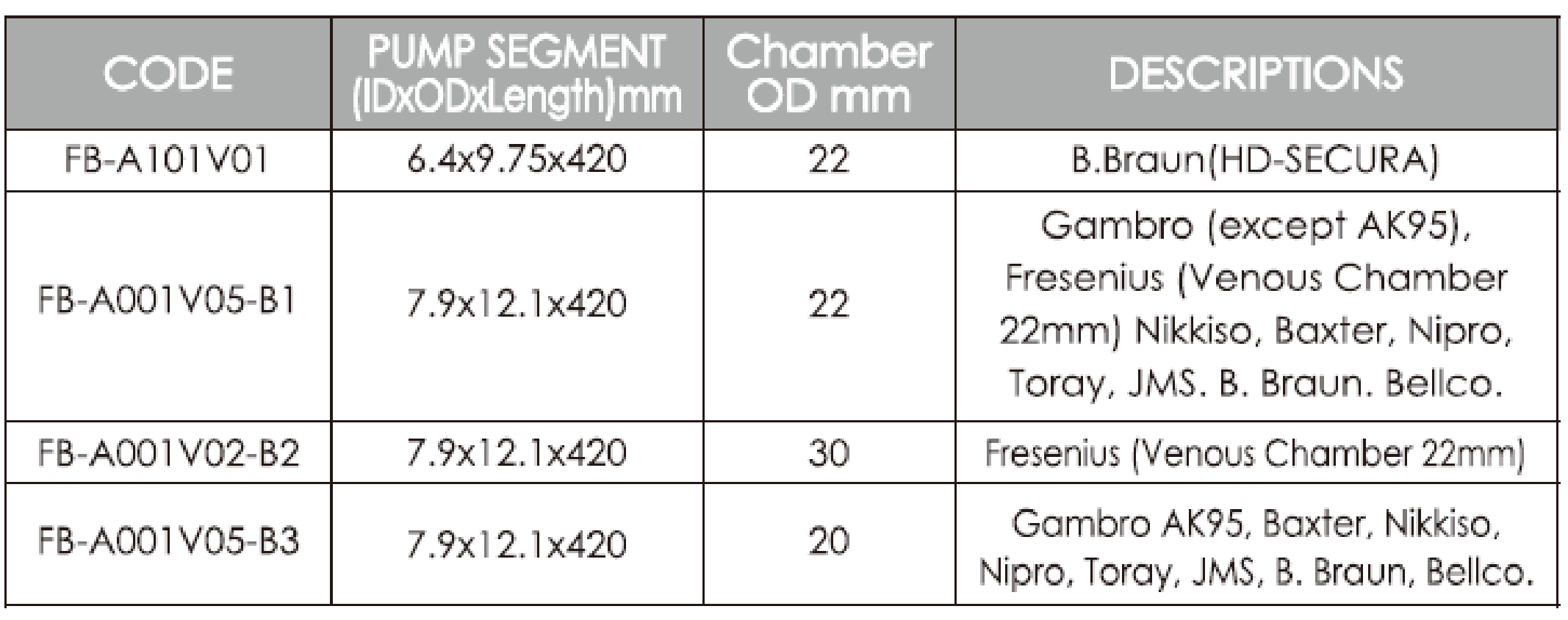
2. The product can be used in conjunction with most of the currently available haemodialysis equipment, including those from brands such as Fresenius, Braun, Gambro, Baxter, Nikkiso, Nipro, Kawasumi, Toray, Bellco, JMS.
3. Single use only. Any resterilization or reuse of the products can result in hazard to the patients.
4. The treatment should be conducted in strict asepsis circumstance.
5. The product is sterilized by Ehtylene Oxide and free from pyrogens.
6. Do not use the product if the protective end caps are not in place or the primary package is damaged.
7. Ensure that the products connect with other medical devices is correct, safe and reliable. If blood leaks, nursing staff should remove obstacle and make adjustment. If it fails, replace the entire blood line.
8. To avoid air embolism caused by the entry of air into the patient's veins, we recommend using air detector.
9. The product is made of medical-grade PVC, which contains DEHP in a concentration that is above 0.1% weight by weight.
10. Attention should be paid when this product is used for pregnant women, breastfeeding mothers, infants and children. Try to use alternatives.
11.Generic names of materials that directly or indirectly contact the fluid path are available to the user upon request.
12.We require that medical devices in combination with Disposable Blood line should be those that have been marked in according with local regulations.
13.Use a 20 gauge or smaller conventional metal needle for accessing the injection ports.
14.Use of incompatible connectors with this blood line may result in blood loss, patient injury or death.
15.Disinfect the blood line using 70% alcohol or 10% Povidone-lodine solution. Other disinfectants intended to be used with this set must be determined as compatible prior to clinical use.
16.Ensure there are no kinks in the bloodline tubing during set-up and patient use.
17.This product can withstand a maximum positive pressure of 100kpa and a maximum negative pressure of 80kpa. During hemodialysis, the maximum blood flow rate is not exceed 300m/min.
18.During hemodialysis treatment, the pressure monitoring tube should be equipped with a transducer protector. The Transducer Protector can prevent the monitor being contaminated by blood. If the transducer protector gets wet with liquid or blood, please replace the transducer protector.
20.Do not expose disposable blood line connections to a lubricant. Exposure could cause connections to separate resulting in patient injury or death.
21.Please disposal the device safely in accordance with local regulations to avoid infection or microbial hazards.
22.If any serious incident that has occurred in relation to the device you should reported to the manufacturer of the device and the competent authority of the Member State in which you are established.